Automatic thickness estimation for skeletal muscle in ultrasonography: evaluation of two enhancement
- 2023-03-03
- 3395
- Guangzhou Sonostar Technologies Co., Limited
Research
Open Access
Automatic thickness estimation for skeletal muscle in ultrasonography: evaluation of two enhancement methods
BioMedical Engineering OnLine 12, Article number: 6 (2013)
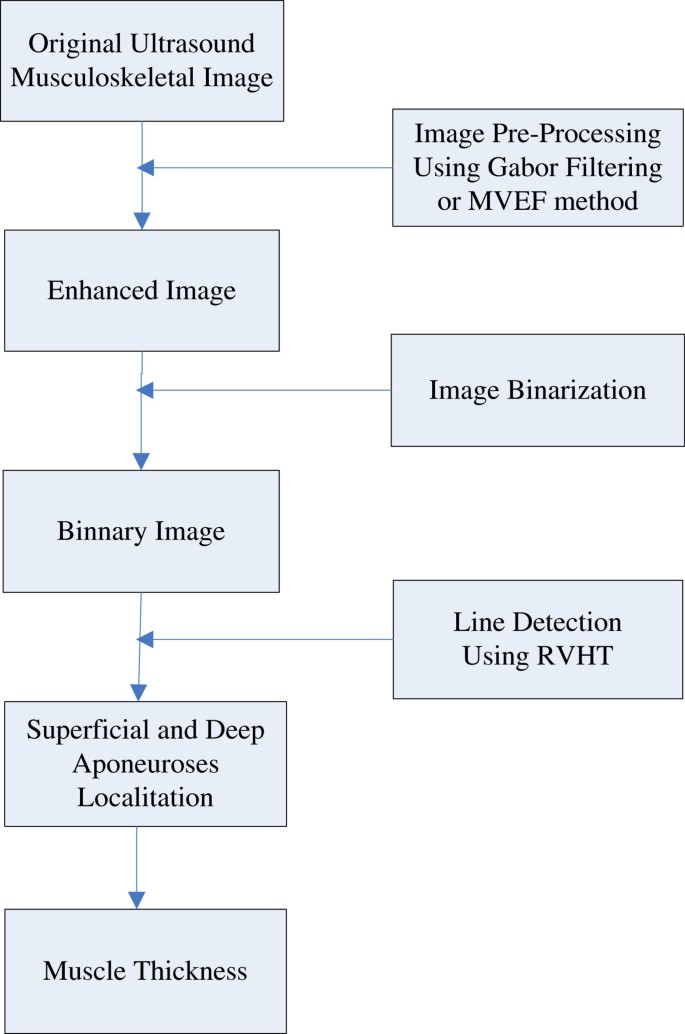
Ultrasonography is a convenient technique to investigate muscle properties and has been widely used to look into muscle functions since it is non-invasive and real-time. Muscle thickness, a quantification which can effectively reflect the muscle activities during muscle contraction, is an important measure for musculoskeletal studies using ultrasonography. The traditional manual operation to read muscle thickness is subjective and time-consuming, therefore a number of studies have focused on the automatic estimation of muscle fascicle orientation and muscle thickness, to which the speckle noises in ultrasound images could be the major obstacle. There have been two popular methods proposed to enhance the hyperechoic regions over the speckles in ultrasonography, namely Gabor Filtering and Multiscale Vessel Enhancement Filtering (MVEF). A study on gastrocnemius muscle is conducted to quantitatively evaluate whether and how these two methods could help the automatic estimation of the muscle thickness based on Revoting Hough Transform (RVHT). The muscle thickness results obtained from each of the two methods are compared with the results from manual measurement, respectively. Data from an aged subject with cerebral infarction is also studied. It’s shown in the experiments that, Gabor Filtering and MVEF can both enable RVHT to generate comparable results of muscle thickness to those by manual drawing (mean ± SD, 1.45 ± 0.48 and 1.38 ± 0.56 mm respectively). However, the MVEF method requires much less computation than Gabor Filtering. Both methods, as preprocessing procedure can enable RVHT the automatic estimation of muscle thickness and MVEF is believed to be a better choice for real-time applications. Ultrasonography, as an important medical image modality in the study of the musculoskeletal system, has been widely used to measure changes in muscle geometry, such as muscle thickness, muscle pennation angle, fascicle length and cross-sectional area[1–8], because it is versatile, inexpensive and radiation-free. More important, the change of muscle properties over time can form signals, representing architectural muscle behavior under contraction, namely sonomyography (SMG)[9–12]. Together with electromyography (EMG)[13–15], SMG can provide quantitative information of muscle contraction, help correlate the actions of several muscles, either agonistic or antagonistic, and subsequently advance medical and engineering efforts to understand, diagnose or even treat musculoskeletal disorders[5, 16–20]. Specifically speaking, muscle thickness (MT), which can effectively reflect the muscle activities during muscle contraction[21–24], is an important measure for musculoskeletal studies using ultrasonography. There have been reports on relationships between MT and many other measures, such as level of physical activity[25], age[26], muscle stiffness[27] and response to exercise[28]. Meanwhile, for major muscles such as quadriceps and gastrocnemius muscle (GM), another important parameter, fascicle length (FL) can hardly be measured directly since it’s beyond the dimension of most ultrasound images. Therefore FL is usually based on MT and pennation angle (for example,[29, 30]). However, MT measurement is traditionally based on manual selection of 2 reference points at the superficial and deep aponeuroses and usage of an on-screen caliper to obtain the MT frame by frame[31–34], which is time-consuming and subjective. There have been several studies targeting at automatic estimation of MT, such as the Revoting Hough Transform (RVHT) method[35, 36], Radon Transform[37], Region of Interest (ROI)[24] and cross-correlation algorithm[38]. Taking RVHT as an example, with assumption that the superficial and deep aponeuroses could be located using RVHT as the very first 2 lines detected, the MT could be computed readily as the distance between them. However, besides the fact that the speckle noise associated with ultrasound image, sarcopenia or loss of muscle mass due to ageing, can affect the quality of the images obtained by ultrasound from deconditioned muscles[20]. This can make it difficult to accurately locate the borders of the muscles to obtain an accurate measure of the MT[39]. There have been two popular methods proposed to enhance the hyperechoic regions over the speckles in ultrasonography, namely Gabor Filtering[36, 40, 41] and Multiscale Vessel Enhancement Filtering (MVEF)[42, 43]. In this paper, to investigate whether image enhancement can make sufficient preparation for automatic estimation of MT and which method is more appropriate for MT estimation, we will evaluate both Gabor Filtering and MVEF on gastrocnemius muscle images. The automatic MT estimation procedure, based on RVHT method proposed previously, could include four steps: 1) image enhancement, 2) thresholding operation to generate black-white image and 3) locating of superficial and deep aponeuroses by RVHT and 4) computation of the distance between aponeuroses. The RVHT first computes accumulator matrix of Hough transform based on a black-white image called an “edge map”. This edge map represents the meaningful image contents. The RVHT method then locates the global maximum in the accumulator matrix of Hough transform that corresponds to the most dominant line-shaped feature points globally, using the standard Hough transform. Then the pixels close to the detected line are removed from the edge map and the Hough transform accumulator matrix is calculated again. The same procedure could be executed to search for another line[35]. For ultrasound images of skeletal muscles, usually the very first two lines detected would be the superficial and deep aponeuroses according to a priori knowledge. Finally the mean distance between each two lines detected by RVHT is computed, and the two lines which have maximum distance between them are recognized as the superficial and deep aponeuroses, and finally the mean distance between them is computed as the MT. Diagram for the procedures is shown in Figure1. The automatic MT estimation procedure. Taking into the account that the fascicles and fibroadipose spetas in a sonogram are tubular and include coherent orientation tendencies, the Gabor Filter bank method can be used to implement the image enhancement. The method includes three steps: the orientation filed estimation, frequency map computation and Gabor filtering confined by the orientation reliability. More details can be consulted in[36, 44]. The Multiscale Vessel Enhancement Filtering method is based on the second order local structure, with excellent noise and background suppression performance. The method also includes three steps: the Hessian matrix estimation (including the choice of Gaussian kernels), computation of eigenvector for each scale and processing for the maximum vesselness response. More details can be found in[42, 43]. Three healthy male subjects (mean ± SD, age = 28.6 ± 0.6 years; body weight 67.0 ± 1.7 kg; height = 1.72 ± 0.01 m) volunteered to participate in this study. No participant had a history of neuromuscular disorders, and all were aware of experimental purposes and procedures. Human subject ethical approval was obtained from the relevant committee from the Hong Kong Polytechnic University, and informed consent was obtained from the subject prior to the experiment. The testing position of the subject was in accordance with the User’s Guide of a Norm dynamometer (Humac/Norm Testing and Rehabilitation System, Computer Sports Medicine, Inc., Massachusetts, USA). The subject was instructed to generate dorsiflexion/plantar-flexion movements in prone position. Th
Abstract
Background
Methods
Results
Conclusions
Background
Methods
Gabor filtering
Multiscale vessel enhancement filtering
Experiments
Subjects
Experiment protocol and data acquisition

















 网站首页
网站首页 产品中心
产品中心 服务支持
服务支持 联系咨询
联系咨询